Technical Bulletin: Detection of E. coli O157:H7 from Whey Protein Concentrate
34 using the BAX® System Real-Time PCR Assay
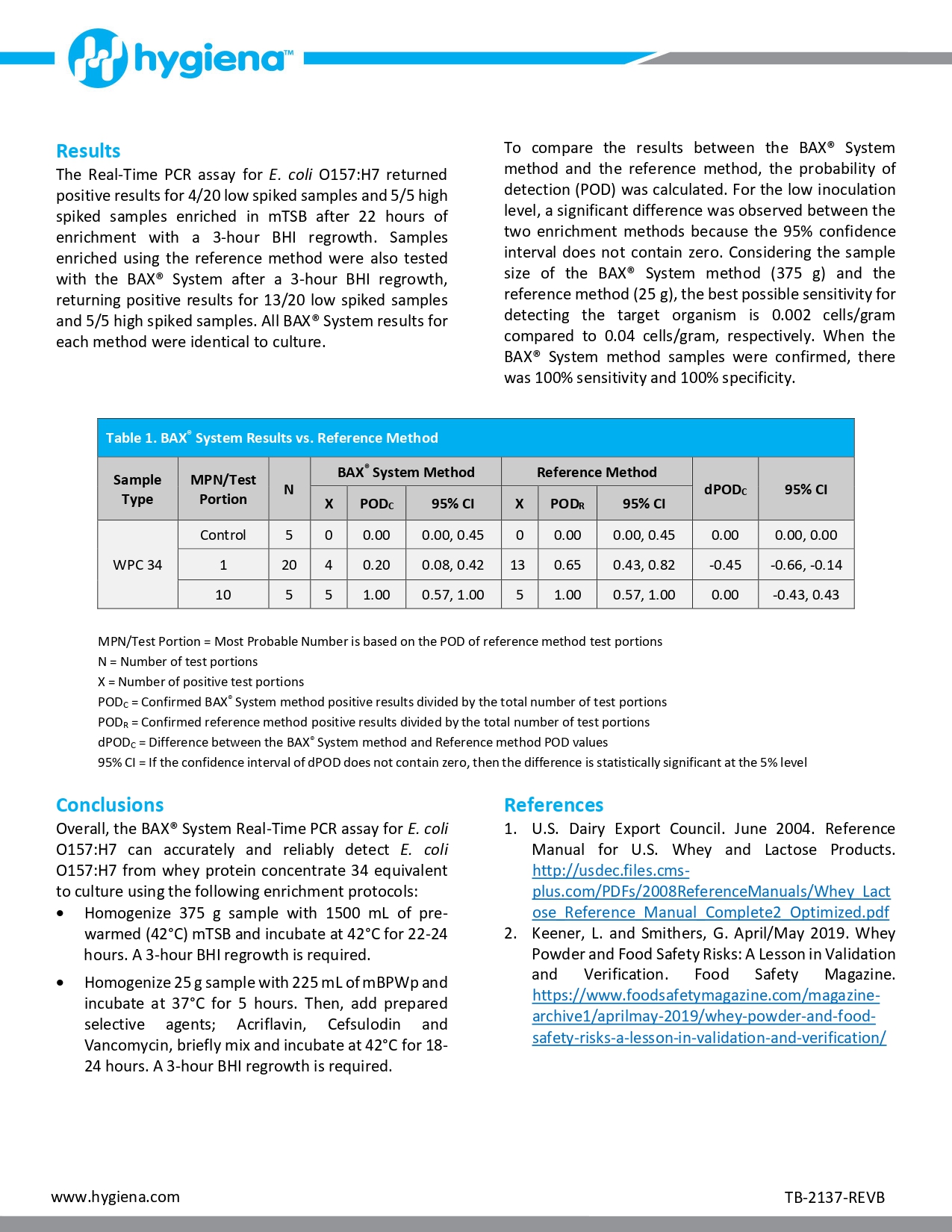
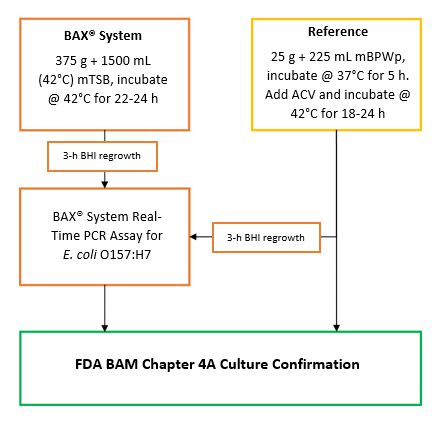
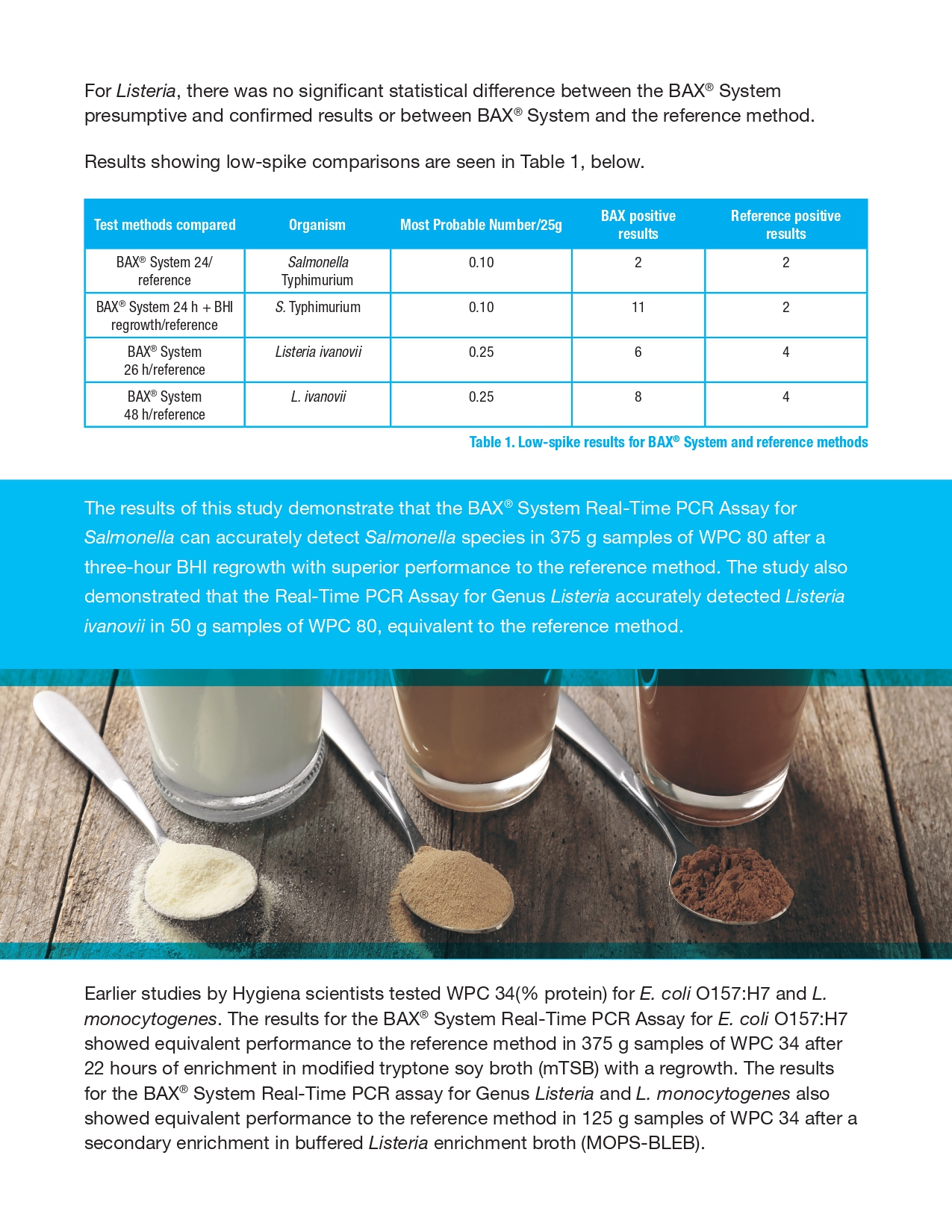
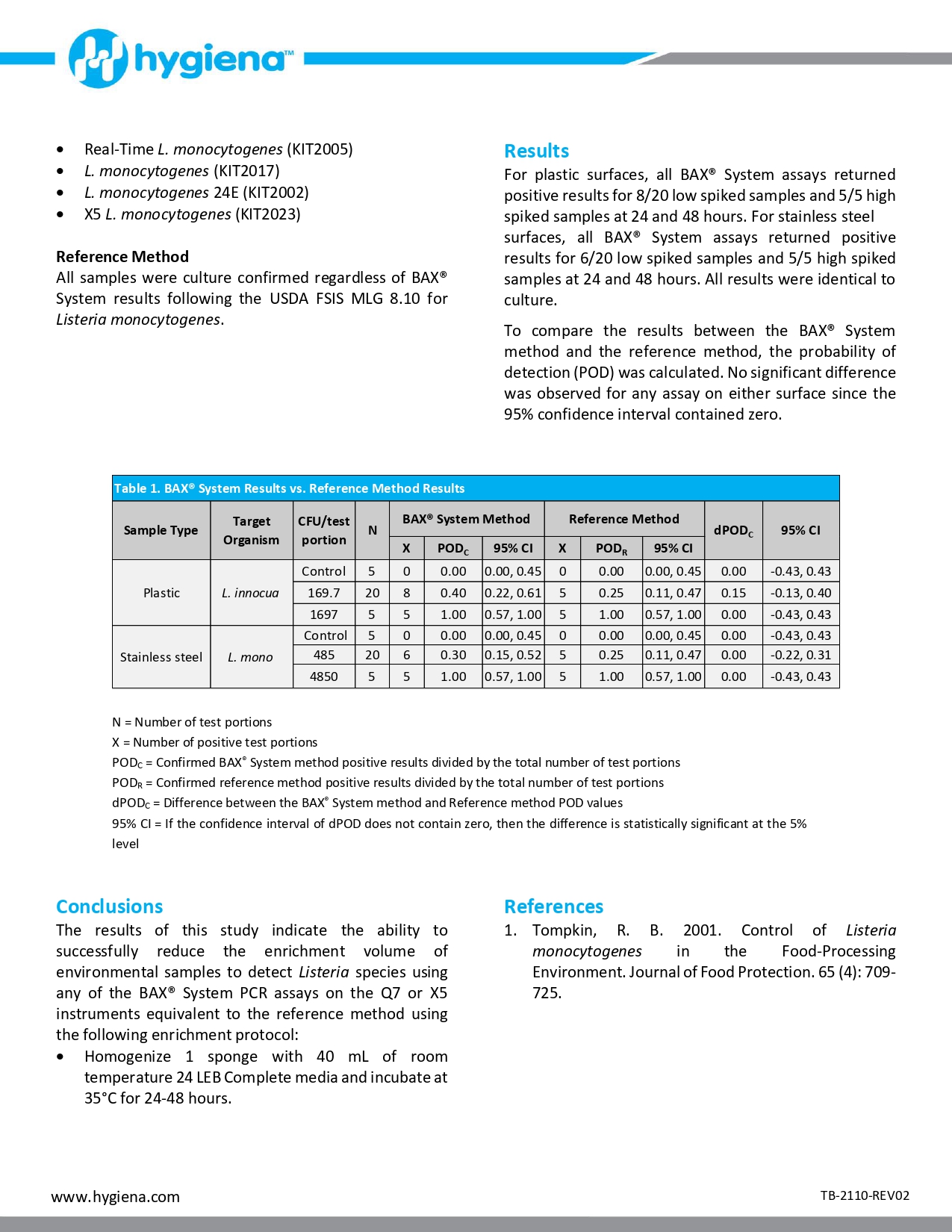
An internal validation study was conducted to measure the method performance of the BAX® System. Real-Time PCR assay for E. coli O157:H7 compared to the U. S. Food and Drug Administration’s Bacteriological Analytical Manual (FDA BAM) reference method for the detection of E. coli O157:H7 in whey protein concentrate 34. Unpaired samples tested in this study were artificially inoculated with concentrations expected to produce low (0.2-2 CFU/test portion) and high (5 CFU/test portion) spike levels. After a 2-week equilibration, samples were enriched and tested. The results were analyzed using the probability of detection (POD), demonstrating complete agreement between presumptive and confirmed results for the BAX® System method and the reference method.
Introduction
Whey-based ingredients are widely used in the food industry to enhance functional and nutritional properties. During processing, whey is pasteurized to eliminate the risk of potential pathogens (1). Nevertheless, in 2018 whey powder was recalled for possible Salmonella contamination (2). Since whey is added to numerous ready-to-eat products, this recall led to additional snack food recalls. Therefore, the control and accurate detection of pathogens during dairy processing is a necessary measure to ensure food safety.










Reviews
There are no reviews yet.